Nerve Pathology Overview
Indications for Nerve Biopsy
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy, when other tests fail to diagnose these conditions. Given that it is an invasive test, however, a nerve biopsy is usually only recommended when the neuropathy is progressive, threatens to become debilitating, and other tests fail to identify a cause.1–3 Vasculitis, amyloidosis, sarcoid and other multifocal disorders may also affect skeletal muscle so that biopsy of muscle in addition to nerve may increase the diagnostic yield.
Location of the Nerve Biopsy
In cases where the neuropathy affects the lower limbs, the superficial peroneal nerve with the peroneus brevis muscle, or the sural nerve with the vastus lateralis or gastrocnemius muscle are usually examined. When only the upper limbs are affected, the superficial radial nerve or a branch of the ulnar nerve in the dorsum of the hand can be biopsied.4–6 Targeted biopsy of a sensory rootlet in demyelinating sensory radiculopathy, or of the obturator nerve branch to the gracilis muscle in multifocal motor neuropathy have also been described.
Pathological Examination
The biopsy nerve samples are prepared in three different ways to fully assess the tissue for diagnostic purposes. One nerve piece (0.5 cm in length) is fixed in 10% formalin and embedded in paraffin; a second piece of similar length is transported in Michel’s medium and frozen in the laboratory using liquid nitrogen; and a third piece (2.0 to 2.5 in length) is placed in glutaraldehyde fixative for preparation of semithin plastic sections and teased myelinated nerve fibers.

Figure 1. Necrotizing arteritis, paraffin section, H&E.
In general, light microscopic examination of formalin-fixed, paraffin-embedded tissue is the most important method for establishing a definitive diagnosis such as vasculitis (Figure 1), amyloidosis (Figure 2A), sarcoidosis, leprosy and lymphoma. If the initial microscopic examination is not informative and these disorders are suspected based on clinical or pathological findings, more extensive sampling of the tissue is indicated to search for a focal definitive lesion. In suspected vasculitis, special stains for the internal elastica of arteries and peri-arterial hemosiderin can provide indirect evidence for the diagnosis in the absence of a definitive lesion, such as inflammatory infiltration of the blood vessel wall or fibrinoid necrosis. All nerves and muscles should be screened by the thioflavin S stain to identify amyloid deposits (Figure 2B). This stain is more sensitive than Congo red for detecting amyloid, but is less specific and requires the Congo red stain to confirm the diagnosis (Figure 2C, 2D). Amyloid can be further subclassified by immunohistochemistry to identify transthyretin or immunoglobulin light chains that cause the two chief types of amyloidotic neuropathy. The immunoreactive transthyretin can be detected in paraffin sections, but staining of light chains requires sections prepared from unfixed frozen tissue. In general, lymphocytic markers are not necessary for diagnosis except for a suspected lymphoma or other lymphoproliferative disorder of nerve.

Figure 2. A: Amyloid deposits (arrows) in endoneurial compartment of nerve, cryosection, H&E. B: Thioflavin S bound to amyloid deposits (arrows), paraffin section (cross-section), fluorescence. C: Amyloid deposits (arrows), paraffin section, Congo Red. D: Outlined area in C enlarged and viewed with crossed polarizing lenses showing greenish birefringence (arrows) in amyloid deposits
Transverse semithin plastic sections of nerve (Figure 3A-D) are more useful for analysis of myelinated fibers than the thicker paraffin sections because of better definition for assessing small myelinated fibers, onion bulbs and other structures. The myelin sheaths in the sections are stained by osmium tetroxide in a pre-embedding step and stained by toluidine blue in a post-embedding step. The findings help to provide a distinction between a purely axonal disorder and primary demyelination, particularly in the acute phase. The number of myelinated fibers per unit area of endoneurium can be quantified, and the result can indicate a loss of myelinated fibers in comparison to reference values if necessary. Reduction of the number of myelinated fibers is usually caused by axonal loss with secondary degeneration of myelin sheaths. The secondary myelin breakdown may be prominent in the acute stage. In the chronic stage, the number of regenerative clusters of three or more small myelinated fibers may be excessive (Figure 3D) and provide further evidence for a chronic axonal process.

Figure 3. Transverse plastic semithin sections of nerve. A: Whole normal sural nerve, toluidine blue. B: Higher magnification of A. The number of small diameter myelinated fibers is roughly twice that of large fibers. C: Neuropathy with an IgM paraprotein reactive to myelin-associated glycoprotein (sural nerve, toluidine blue). There is a loss of myelinated fibers and scattered isolated thinly myelinated fibers, probably reflecting segmental remyelination. A few onion bulbs are observed (arrows). D: Multifocal motor neuropathy. A transverse section of the motor nerve of the gracilis muscle displays large regenerative clusters of small myelinated fibers (arrows) (toluidine blue-basic fuchsin).
Acute primary demyelination of nerve is represented by large “naked” axons that lack myelin sheaths and are often encircled by myelin debris-containing macrophages. Additional evidence for demyelination can be provided by electron microscopy studies or distinctive ultrastructural changes such as widened myelin lamellae in neuropathy associated with monoclonal IgM anti-MAG (myelin-associated glycoprotein) antibodies (Figure 4). Months after the clinical onset, onion bulbs may appear in transverse sections (Figure 3C). The abnormality consists of concentrically oriented Schwann cell processes that surround a thinly-myelinated fiber as a result of segmental remyelination. Segmental remyelination appears more often without onion bulb formation, but it cannot be distinguished reliably from fibers that have undergone axonal regeneration and remyelination in semithin sections. This distinction requires teased myelinated fiber analysis (Figure 5), which is more sensitive than semithin sections for identifying demyelinating and axonal features. Segmental demyelination, segmental remyelination and tomacula (focally thickened myelin sheaths) are characteristic features of a demyelinating disorder, whereas myelin breakdown into ovoids along the full length of a teased fiber indicates axonal degeneration. The analysis requires quantifying the abnormalities in a minimum of 50 teased myelinated fibers with comparison to the data of age-matched normal subjects. However, the teased fiber results in chronic neuropathies can be difficult to interpret because demyelination can result from either primary attack of the myelin sheath or a secondary effect of axonal dysfunction.

Figure 5. A: A single teased myelinated fiber shows segmental remyelination in the form of three short segments (arrows) of myelin flanked by normal internodes (osmium tetroxide). B: Higher magnification of outlined area in A
Diagnostic Yield
Several studies have been published that reviewed the authors’ experience with nerve biopsies. Argov et al (1989) reported that the nerve biopsy contributed to the final diagnosis in 18% of 120 biopsies. Neundorfer et al (1990) reported that the diagnosis was made on basis of the biopsy alone in 27% of 56 patients and that it contributed valuable diagnostic information in 37%.7 Oh (1990) reported that specific diagnoses were made in 24% of 385 cases, and that the biopsy provided helpful information in 45%. Chia et al (1996) reported that the biopsy was necessary for diagnosis in 33% of 100 elderly patients over the age of 65.8 Gabriel et al (2000) reported that the biopsy changed the diagnosis in 14% of 50 cases, and was helpful in an additional 70% as it confirmed the suspected diagnosis.9 Midroni and Bilboa (2006) reported that the biopsy was essential for patient management in 21% of 234 cases. Schweikert et al (2007) reported that the biopsy was diagnostic in 16% of 38 patients, and supportive of the suspected diagnosis in 21%.10 The differences between studies probably resulted from differences in patient selection between the reporting centers.
Vasculitis
Vasculitis was by far the most common diagnosis made on the basis of nerve biopsy (Figure 1). Vasculitis was identified in 12% of biopsies reported by Oh (1990), 8% of patients reported by Midroni and Bilbao (2006), 16% of cases reported by Schweikert et al (2007), and 33% of elderly patients reported by Chia et al (1996).
Most authors found that doing a muscle biopsy added to the diagnostic yield. Chia et al (1996) reported that of the patients with vasculitis, 43% had vasculitic changes in both nerve and muscle, 65% in muscle, and 78% in nerves. Schroder et al (1998) reported that of 129 cases of vasculitis, both muscle and nerve were positive in 27%, nerve only in 30%, and muscle only in 27%. Seo et al (2004) reported that the muscle biopsy was necessary for diagnosis in 12% of their patients.11 Vital et al (2006) reported that muscle biopsy improved the yield of definite vasculitis by 27%.12 Vrancken et al (2010) reported that in patients diagnosed with vasculitis, the additional yield of muscle biopsy was 15%.13 Bennett et al (2008), reported that combined sural nerve and vastus lateralis muscle biopsy did not significantly increase the diagnostic yield compared to nerve biopsy alone.14 However, they recommended that if the superficial peroneal nerve is chosen, then it would be appropriate to also biopsy the peroneus brevis muscle.
Clinically, vasculitis in neuropathy can be systemic and involve other organs, or nonsystemic in which case it is clinically localized to peripheral nerves (Figure 1). In systemic vasculitis, the diagnosis can be based on biopsy findings of any affected organ, but in nonsystemic vasculitic neuropathy, the diagnosis is made on the basis of nerve and muscle biopsy.15–19 Patients presented with mononeuritis multiplex or diffuse polyneuropathy in an asymmetric or distal and symmetric distribution, probably resulting from a confluence of lesions. In a large series of nonsystemic vasculitic neuropathy, asymmetric findings were reported in 59% to 98% of cases, with pain being a prominent feature in 75% to 98%.16
In the series by Chia et al (1996), 23% of biopsies had a necrotizing arteritis, and 15% had inflammatory infiltrates in the vicinity of nerve and muscle blood vessels associated with axonal degeneration and multifocal neuropathy. In 20 of 33 patients, the neuropathy occurred in the context of a multisystem disorder and in 13 the biopsy was the only indication of vasculitis.
In electrophysiologic studies, Zivkovic et al (2007) reported that the most common presentation of patients with vasculitic neuropathy was generalized neuropathy with electrodiagnosis showing a pattern of mononeuritis multiplex in 27.5%, and axonal sensorimotor neuropathy with side to side amplitude asymmetry in 50%, with the remaining showing a symmetric axonal neuropathy.20 Seo et al (2004) reported that 16 of their 106 patients (16%) with vasculitic neuropathy presented with a purely sensory axonal neuropathy, and the disorder was symmetric in 53% of them.
Pathological changes suggestive of vasculitis without frank vasculitic lesions have also been described in some patients with progressive idiopathic axonal neuropathy (Vrancken et al, 2004), and Logigian et al (1993), and Kelkar et al (2002) reported finding focal inflammatory infiltrates without necrosis of blood vessel walls in some patients presenting with sensory neuropathy or multifocal neuropathy that responded to immune therapy.21-23
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) and Other Demyelinating Polyneuropathies
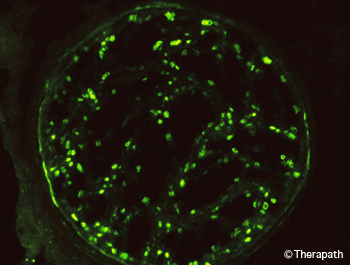
Figure 6. The C3d component of complement in the myelin sheaths of nerve fibers is demonstrated by immunofluorescence. This is accompanied by the presence of IgM at the same site (not shown).
In published nerve biopsy studies, CIDP was diagnosed in 12% of cases reported by Oh (1990), 14% of patients reported by Chia et al (1996), and 5% of cases reported by Schweikert et al (2007). Logigian et al (1994) reported identifying CIDP in 16 of 105 patients (15%) who underwent nerve biopsy.24
The diagnosis of CIDP requires evidence for primary demyelination, which can be provided by nerve conduction studies or pathological examination of nerve biopsy. Electrodiagnostic studies are preferred, as they are minimally invasive, and convenient. However, there is a discrepancy between their sensitivity and specificity, so that the diagnosis of CIDP may sometimes be missed.25,26 In such cases, the demyelination may be detected by nerve biopsy. Biopsy is particularly useful in sensory CIDP (Chin et al, 2004) as electrodiagnostic measures of demyelination are applicable mostly to motor nerves and they cannot be determined in nerves that are unresponsive to stimulation.27 The discrepancy between sensitivity and specificity of electrodiagnostic studies results from a number of factors including: 1) demyelinating changes in sensory nerves are not readily detected, 2) demyelination can occur proximally or distally to the nerve segments examined, 3) demyelinating changes can be masked by axonal degeneration, and 4) early or mild demyelinating changes may not reach diagnostic threshold.28 The latter results from the fact that nerve conduction abnormalities were originally considered to be in the demyelinating range if they exceeded those that can result from axonal degeneration, with axonal neuropathy being the default diagnosis.
Accordingly, Logigian et al (1994) reported that, based on nerve biopsy or nerve conduction studies, the presence of CIDP would have been missed in 5 of 16 patients with CIDP if it weren’t for the nerve biopsy. Of these cases, 3 had sensory CIDP with absent sural responses and sparing of the motor nerves, and 2 had indeterminate studies, with absent responses in the legs. Bosboom et al (2001) reported considerable overlap between nerve biopsy abnormalities in patients classified as having CIDP or chronic axonal neuropathy, but the classification was based on AAN electrodiagnostic demyelinating criteria that have a sensitivity of only 45% (Rajabally et al, 2009), putting their conclusions into question.26,29 Haq et al (2000) reported that sural nerve histologic criteria increased the diagnostic yield when added to the electrodiagnostic criteria for CIDP. Vallat et al (2003) reported that 8 of 44 biopsied patients with pathological diagnosis of CIDP, including 5 that responded to immune therapy, had electrodiagnostic studies that did not meet any of the accepted criteria for demyelination.30 They concluded that a significant number of patients with CIDP are erroneously classified as having axonal neuropathy based on EDX criteria alone. Chin et al (2004), reported 8 patients with sensory neuropathy and axonal or minimally abnormal electrodiagnostic changes, in whom CIDP was diagnosed by sural nerve biopsy.
In addition to distinguishing between demyelination and axonal degeneration, nerve biopsy can help diagnose neuropathy associated with anti-MAG monoclonal IgM antibodies by the presence of IgM and complement on myelin sheaths (Figure 6) and widened myelin lamellae (Figure 4).31 It can also help differentiate between multifocal motor neuropathy and motor neuron disease by the presence of regenerative clusters in motor nerves of patients with multifocal motor neuropathy (Figure 3D).32,33 Studies are ongoing to identify markers that would distinguish between inflammatory and non-inflammatory demyelinating neuropathies.34–36
Other Diagnoses
Amyloid, sarcoidosis, leprosy, or lymphomatosis were found in an occasional patient in several of the series.1,2,8–10 These findings were generally not suspected beforehand. In the series of sarcoid neuropathy by Said et al (2002b), 9 of 11 patients were not known to have sarcoidosis prior to the biopsy. Amyloid neuropathy can result from deposition of immunoglobulin light chain fragments in primary amyloidosis, or of mutated transthyretin, apolipoprotein A-1 or gelsolin in familial forms of amyloidosis. In primary amyloidosis, 90% of patients have a serum or urine monoclonal gammopathy. The type of amyloid deposits can be identified by immunocytochemistry in primary amyloidosis and in familial amyloidotic neuropathy due to a mutation of transthyretin.37–39
Potential Complications of Nerve Biopsy
The rate of complications varied widely between centers. In chronological order, Asbury and Connolly (1973) reported serious side effects in 2 of 103 cases, one with neuroma and one with pain.40 Stevens et al (1975) reported that 10% had significant pain or paresthesias at one year.41 Pollock et al (1983) reported that at 5 years patients only reported non-troublesome and mild dysesthetic symptoms.42 Dyck et al (1984) reported persistent discomfort in 36% and 40% after 3 and 6 months respectively, with 10% having significant pain or paresthesias after one year. Solders et al (1988) reported that immediately after the surgery, approximately half the patients (26/54) experience pain in the operated area.43 In 6/54 (11%) the pain lasted for more than 7 days, and at 6 months 3 of the patients (5.5%) had moderate discomfort and 3 severe discomfort described as a pinprick or “tight” sensation during standing or walking. Some loss of sensation in the foot was reported by 24/54 (44%) of the patients. Neundorfer et al (1990) reported that 53% had sensory deficits, 32% had paresthesias and dysesthesias, and 28% had pain, which in 25% was present at 21 months. Oh (1990) reported that 0.5% had neuralgia lasting one year. Gabriel et al (2000) reported that at 6 months, 20% reported pain at the biopsy site. Dahlin et al (1997) reported that 40% of patients with diabetes but none of the patients without diabetes reported pain in the biopsy site at 20 to 44 months after the biopsy.44 Theriault et al (1998) reported that at 12 months, 19% had allodynia that was rated as 3 on a scale of 1 to 10.45 Said (2002a) recommended immobilizing the leg in a plastic cast for 10 days following surgery to prevent excessive tension.
- Oh S. Diagnostic usefulness and limitations of the sural nerve biopsy. Yonsei Med J. 1990;31(1):1-26. [PubMed]
- Midroni G, Bilbao J. Nerve Biopsy. In: Peripheral Nerve Diseases, Handbook of Clinical Neurophysiology. Vol 7. Elsevier BV; 2006:63-94.
- Schröder J. Recommendations for the examination of peripheral nerve biopsies. Virchows Arch. 1998;432(3):199-205. [PubMed]
- Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59(10):1532-1535. [PubMed]
- Ruth A, Schulmeyer F, Roesch M, Woertgen C, Brawanski A. Diagnostic and therapeutic value due to suspected diagnosis, long-term complications, and indication for sural nerve biopsy. Clin Neurol Neurosurg. 2005;107(3):214-217. [PubMed]
- Bevilacqua N, Rogers L, Malik R, Armstrong D. Technique of the sural nerve biopsy. J Foot Ankle Surg. 2007;46(2):139-142. [PubMed]
- Neundörfer B, Grahmann F, Engelhardt A, Harte U. Postoperative effects and value of sural nerve biopsies: a retrospective study. Eur Neurol. 1990;30(6):350-352. [PubMed]
- Chia L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain. 1996;119 ( Pt 4):1091-1098. [PubMed]
- Gabriel C, Howard R, Kinsella N, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry. 2000;69(4):442-446. [PubMed]
- Schweikert K, Fuhr P, Probst A, Tolnay M, Renaud S, Steck A. Contribution of nerve biopsy to unclassified neuropathy. Eur Neurol. 2007;57(2):86-90. [PubMed]
- Seo J, Ryan H, Claussen G, Thomas T, Oh S. Sensory neuropathy in vasculitis: a clinical, pathologic, and electrophysiologic study. Neurology. 2004;63(5):874-878. [PubMed]
- Vital C, Vital A, Canron M, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst. 2006;11(1):20-29. [PubMed]
- Vrancken A, Gathier C, Cats E, Notermans N, Collins M. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18(1):49-58. [PubMed]
- Bennett D, Groves M, Blake J, et al. The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry. 2008;79(12):1376-1381. [PubMed]
- Collins M. Localized vasculitis and the peripheral nervous system. J Rheumatol. 2005;32(5):769-772. [PubMed]
- Collins M, Mendell J, Periquet M, et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55(5):636-643. [PubMed]
- Sugiura M, Koike H, Iijima M, et al. Clinicopathologic features of nonsystemic vasculitic neuropathy and microscopic polyangiitis-associated neuropathy: a comparative study. J Neurol Sci. 2006;241(1-2):31-37. [PubMed]
- Burns T, Schaublin G, Dyck P. Vasculitic neuropathies. Neurol Clin. 2007;25(1):89-113. [PubMed]
- Gorson K. Vasculitic neuropathies: an update. Neurologist. 2007;13(1):12-19. [PubMed]
- Zivković S, Ascherman D, Lacomis D. Vasculitic neuropathy–electrodiagnostic findings and association with malignancies. Acta Neurol Scand. 2007;115(6):432-436. [PubMed]
- Vrancken A, Notermans N, Jansen G, Wokke J, Said G. Progressive idiopathic axonal neuropathy–a comparative clinical and histopathological study with vasculitic neuropathy. J Neurol. 2004;251(3):269-278. [PubMed]
- Logigian E, Shefner J, Frosch M, et al. Nonvasculitic, steroid-responsive mononeuritis multiplex. Neurology. 1993;43(5):879-883. [PubMed]
- Kelkar P, McDermott W, Parry G. Sensory-predominant, painful, idiopathic neuropathy: inflammatory changes in sural nerves. Muscle Nerve. 2002;26(3):413-416. [PubMed]
- Logigian E, Kelly J, Adelman L. Nerve conduction and biopsy correlation in over 100 consecutive patients with suspected polyneuropathy. Muscle Nerve. 1994;17(9):1010-1020. [PubMed]
- De S, Chin R, Sander H, Latov N, Brannagan T. Demyelinating findings in typical and atypical chronic inflammatory demyelinating polyneuropathy: sensitivity and specificity. J Clin Neuromuscul Dis. 2009;10(4):163-169. [PubMed]
- Rajabally Y, Nicolas G, Piéret F, Bouche P, Van den. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: a multicentre European study. J Neurol Neurosurg Psychiatry. 2009;80(12):1364-1368. [PubMed]
- Chin R, Latov N, Sander H, et al. Sensory CIDP presenting as cryptogenic sensory polyneuropathy. J Peripher Nerv Syst. 2004;9(3):132-137. [PubMed]
- Krarup C, Trojaborg W. Sensory pathophysiology in chronic acquired demyelinating neuropathy. Brain. 1996;119 ( Pt 1):257-270. [PubMed]
- Bosboom W, van den, Franssen H, et al. Diagnostic value of sural nerve demyelination in chronic inflammatory demyelinating polyneuropathy. Brain. 2001;124(Pt 12):2427-2438. [PubMed]
- Vallat J, Tabaraud F, Magy L, et al. Diagnostic value of nerve biopsy for atypical chronic inflammatory demyelinating polyneuropathy: evaluation of eight cases. Muscle Nerve. 2003;27(4):478-485. [PubMed]
- Takatsu M, Hays AP, Latov N, et al. Immunofluorescence study of patients with neuropathy and IgM M proteins. A. 1985;18(2):173-181. doi:10.1002/ana.410180203
- Corbo M, Abouzahr M, Latov N, et al. Motor nerve biopsy studies in motor neuropathy and motor neuron disease. Muscle Nerve. 1997;20(1):15-21. [PubMed]
- Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1):197-201. [PubMed]
- Renaud S, Hays A, Brannagan T, et al. Gene expression profiling in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2005;159(1-2):203-214. [PubMed]
- Sommer C, Brandner S, Dyck P, et al. 147th ENMC international workshop: guideline on processing and evaluation of sural nerve biopsies, 15-17 December 2006, Naarden, The Netherlands. Neuromuscul Disord. 2008;18(1):90-96. [PubMed]
- Kinter J, Broglio L, Steck A, et al. Gene expression profiling in nerve biopsy of vasculitic neuropathy. J Neuroimmunol. 2010;225(1-2):184-189. [PubMed]
- Kissel J, Mendell J. Neuropathies associated with monoclonal gammopathies. Neuromuscul Disord. 1996;6(1):3-18. [PubMed]
- Matsuda M, Gono T, Morita H, Katoh N, Kodaira M, Ikeda S. Peripheral nerve involvement in primary systemic AL amyloidosis: a clinical and electrophysiological study. Eur J Neurol. 2011;18(4):604-610. [PubMed]
- Cappellari M, Cavallaro T, Ferrarini M, et al. Variable presentations of TTR-related familial amyloid polyneuropathy in seventeen patients. J Peripher Nerv Syst. 2011;16(2):119-129. [PubMed]
- Asbury A, Connolly E. Sural nerve biopsy. Technical note. J Neurosurg. 1973;38(3):391-392. [PubMed]
- Stevens J, Lofgren E, Dyck P. Biopsy of peripheral nerves. In: Peripheral Neuropathy. Vol 1. Philadelphia: Saunders; 1975:410-423.
- Pollock M, Nukada H, Taylor P, Donaldson I, Carroll G. Comparison between fascicular and whole sural nerve biopsy. Ann Neurol. 1983;13(1):65-68. [PubMed]
- Solders G. Discomfort after fascicular sural nerve biopsy. Acta Neurol Scand. 1988;77(6):503-504. [PubMed]
- Dahlin L, Eriksson K, Sundkvist G. Persistent postoperative complaints after whole sural nerve biopsies in diabetic and non-diabetic subjects. Diabet Med. 1997;14(5):353-356. [PubMed]
- Theriault M, Dort J, Sutherland G, Zochodne D. A prospective quantitative study of sensory deficits after whole sural nerve biopsies in diabetic and nondiabetic patients. Surgical approach and the role of collateral sprouting. Neurology. 1998;50(2):480-484. [PubMed]
Related Articles
Nerve Pathology Overview
Becky Tsai2022-09-04T01:43:54-05:00
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy... [Read More]
Muscle Pathology Overview
Becky Tsai2022-09-04T14:24:50-05:00
Muscle biopsy is used to confirm that there is a morphological or biochemical abnormality that correlates with or could account for clinical symptoms. [Read More]
Therapath Neuropathology Announces National Ophthalmic Pathology Program
Becky Tsai2022-09-05T17:49:10-05:00
Therapath LLC, a leader in neuropathology laboratory services, launches its national ophthalmic pathology program. [Read More]
Nerve Pathology Overview
Indications for Nerve Biopsy
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy, when other tests fail to diagnose these conditions. Given that it is an invasive test, however, a nerve biopsy is usually only recommended when the neuropathy is progressive, threatens to become debilitating, and other tests fail to identify a cause.1–3 Vasculitis, amyloidosis, sarcoid and other multifocal disorders may also affect skeletal muscle so that biopsy of muscle in addition to nerve may increase the diagnostic yield.
Location of the Nerve Biopsy
In cases where the neuropathy affects the lower limbs, the superficial peroneal nerve with the peroneus brevis muscle, or the sural nerve with the vastus lateralis or gastrocnemius muscle are usually examined. When only the upper limbs are affected, the superficial radial nerve or a branch of the ulnar nerve in the dorsum of the hand can be biopsied.4–6 Targeted biopsy of a sensory rootlet in demyelinating sensory radiculopathy, or of the obturator nerve branch to the gracilis muscle in multifocal motor neuropathy have also been described.
Pathological Examination
The biopsy nerve samples are prepared in three different ways to fully assess the tissue for diagnostic purposes. One nerve piece (0.5 cm in length) is fixed in 10% formalin and embedded in paraffin; a second piece of similar length is transported in Michel’s medium and frozen in the laboratory using liquid nitrogen; and a third piece (2.0 to 2.5 in length) is placed in glutaraldehyde fixative for preparation of semithin plastic sections and teased myelinated nerve fibers.

Figure 1. Necrotizing arteritis, paraffin section, H&E.
In general, light microscopic examination of formalin-fixed, paraffin-embedded tissue is the most important method for establishing a definitive diagnosis such as vasculitis (Figure 1), amyloidosis (Figure 2A), sarcoidosis, leprosy and lymphoma. If the initial microscopic examination is not informative and these disorders are suspected based on clinical or pathological findings, more extensive sampling of the tissue is indicated to search for a focal definitive lesion. In suspected vasculitis, special stains for the internal elastica of arteries and peri-arterial hemosiderin can provide indirect evidence for the diagnosis in the absence of a definitive lesion, such as inflammatory infiltration of the blood vessel wall or fibrinoid necrosis. All nerves and muscles should be screened by the thioflavin S stain to identify amyloid deposits (Figure 2B). This stain is more sensitive than Congo red for detecting amyloid, but is less specific and requires the Congo red stain to confirm the diagnosis (Figure 2C, 2D). Amyloid can be further subclassified by immunohistochemistry to identify transthyretin or immunoglobulin light chains that cause the two chief types of amyloidotic neuropathy. The immunoreactive transthyretin can be detected in paraffin sections, but staining of light chains requires sections prepared from unfixed frozen tissue. In general, lymphocytic markers are not necessary for diagnosis except for a suspected lymphoma or other lymphoproliferative disorder of nerve.

Figure 2. A: Amyloid deposits (arrows) in endoneurial compartment of nerve, cryosection, H&E. B: Thioflavin S bound to amyloid deposits (arrows), paraffin section (cross-section), fluorescence. C: Amyloid deposits (arrows), paraffin section, Congo Red. D: Outlined area in C enlarged and viewed with crossed polarizing lenses showing greenish birefringence (arrows) in amyloid deposits
Transverse semithin plastic sections of nerve (Figure 3A-D) are more useful for analysis of myelinated fibers than the thicker paraffin sections because of better definition for assessing small myelinated fibers, onion bulbs and other structures. The myelin sheaths in the sections are stained by osmium tetroxide in a pre-embedding step and stained by toluidine blue in a post-embedding step. The findings help to provide a distinction between a purely axonal disorder and primary demyelination, particularly in the acute phase. The number of myelinated fibers per unit area of endoneurium can be quantified, and the result can indicate a loss of myelinated fibers in comparison to reference values if necessary. Reduction of the number of myelinated fibers is usually caused by axonal loss with secondary degeneration of myelin sheaths. The secondary myelin breakdown may be prominent in the acute stage. In the chronic stage, the number of regenerative clusters of three or more small myelinated fibers may be excessive (Figure 3D) and provide further evidence for a chronic axonal process.

Figure 3. Transverse plastic semithin sections of nerve. A: Whole normal sural nerve, toluidine blue. B: Higher magnification of A. The number of small diameter myelinated fibers is roughly twice that of large fibers. C: Neuropathy with an IgM paraprotein reactive to myelin-associated glycoprotein (sural nerve, toluidine blue). There is a loss of myelinated fibers and scattered isolated thinly myelinated fibers, probably reflecting segmental remyelination. A few onion bulbs are observed (arrows). D: Multifocal motor neuropathy. A transverse section of the motor nerve of the gracilis muscle displays large regenerative clusters of small myelinated fibers (arrows) (toluidine blue-basic fuchsin).
Acute primary demyelination of nerve is represented by large “naked” axons that lack myelin sheaths and are often encircled by myelin debris-containing macrophages. Additional evidence for demyelination can be provided by electron microscopy studies or distinctive ultrastructural changes such as widened myelin lamellae in neuropathy associated with monoclonal IgM anti-MAG (myelin-associated glycoprotein) antibodies (Figure 4). Months after the clinical onset, onion bulbs may appear in transverse sections (Figure 3C). The abnormality consists of concentrically oriented Schwann cell processes that surround a thinly-myelinated fiber as a result of segmental remyelination. Segmental remyelination appears more often without onion bulb formation, but it cannot be distinguished reliably from fibers that have undergone axonal regeneration and remyelination in semithin sections. This distinction requires teased myelinated fiber analysis (Figure 5), which is more sensitive than semithin sections for identifying demyelinating and axonal features. Segmental demyelination, segmental remyelination and tomacula (focally thickened myelin sheaths) are characteristic features of a demyelinating disorder, whereas myelin breakdown into ovoids along the full length of a teased fiber indicates axonal degeneration. The analysis requires quantifying the abnormalities in a minimum of 50 teased myelinated fibers with comparison to the data of age-matched normal subjects. However, the teased fiber results in chronic neuropathies can be difficult to interpret because demyelination can result from either primary attack of the myelin sheath or a secondary effect of axonal dysfunction.

Figure 5. A: A single teased myelinated fiber shows segmental remyelination in the form of three short segments (arrows) of myelin flanked by normal internodes (osmium tetroxide). B: Higher magnification of outlined area in A
Diagnostic Yield
Several studies have been published that reviewed the authors’ experience with nerve biopsies. Argov et al (1989) reported that the nerve biopsy contributed to the final diagnosis in 18% of 120 biopsies. Neundorfer et al (1990) reported that the diagnosis was made on basis of the biopsy alone in 27% of 56 patients and that it contributed valuable diagnostic information in 37%.7 Oh (1990) reported that specific diagnoses were made in 24% of 385 cases, and that the biopsy provided helpful information in 45%. Chia et al (1996) reported that the biopsy was necessary for diagnosis in 33% of 100 elderly patients over the age of 65.8 Gabriel et al (2000) reported that the biopsy changed the diagnosis in 14% of 50 cases, and was helpful in an additional 70% as it confirmed the suspected diagnosis.9 Midroni and Bilboa (2006) reported that the biopsy was essential for patient management in 21% of 234 cases. Schweikert et al (2007) reported that the biopsy was diagnostic in 16% of 38 patients, and supportive of the suspected diagnosis in 21%.10 The differences between studies probably resulted from differences in patient selection between the reporting centers.
Vasculitis
Vasculitis was by far the most common diagnosis made on the basis of nerve biopsy (Figure 1). Vasculitis was identified in 12% of biopsies reported by Oh (1990), 8% of patients reported by Midroni and Bilbao (2006), 16% of cases reported by Schweikert et al (2007), and 33% of elderly patients reported by Chia et al (1996).
Most authors found that doing a muscle biopsy added to the diagnostic yield. Chia et al (1996) reported that of the patients with vasculitis, 43% had vasculitic changes in both nerve and muscle, 65% in muscle, and 78% in nerves. Schroder et al (1998) reported that of 129 cases of vasculitis, both muscle and nerve were positive in 27%, nerve only in 30%, and muscle only in 27%. Seo et al (2004) reported that the muscle biopsy was necessary for diagnosis in 12% of their patients.11 Vital et al (2006) reported that muscle biopsy improved the yield of definite vasculitis by 27%.12 Vrancken et al (2010) reported that in patients diagnosed with vasculitis, the additional yield of muscle biopsy was 15%.13 Bennett et al (2008), reported that combined sural nerve and vastus lateralis muscle biopsy did not significantly increase the diagnostic yield compared to nerve biopsy alone.14 However, they recommended that if the superficial peroneal nerve is chosen, then it would be appropriate to also biopsy the peroneus brevis muscle.
Clinically, vasculitis in neuropathy can be systemic and involve other organs, or nonsystemic in which case it is clinically localized to peripheral nerves (Figure 1). In systemic vasculitis, the diagnosis can be based on biopsy findings of any affected organ, but in nonsystemic vasculitic neuropathy, the diagnosis is made on the basis of nerve and muscle biopsy.15–19 Patients presented with mononeuritis multiplex or diffuse polyneuropathy in an asymmetric or distal and symmetric distribution, probably resulting from a confluence of lesions. In a large series of nonsystemic vasculitic neuropathy, asymmetric findings were reported in 59% to 98% of cases, with pain being a prominent feature in 75% to 98%.16
In the series by Chia et al (1996), 23% of biopsies had a necrotizing arteritis, and 15% had inflammatory infiltrates in the vicinity of nerve and muscle blood vessels associated with axonal degeneration and multifocal neuropathy. In 20 of 33 patients, the neuropathy occurred in the context of a multisystem disorder and in 13 the biopsy was the only indication of vasculitis.
In electrophysiologic studies, Zivkovic et al (2007) reported that the most common presentation of patients with vasculitic neuropathy was generalized neuropathy with electrodiagnosis showing a pattern of mononeuritis multiplex in 27.5%, and axonal sensorimotor neuropathy with side to side amplitude asymmetry in 50%, with the remaining showing a symmetric axonal neuropathy.20 Seo et al (2004) reported that 16 of their 106 patients (16%) with vasculitic neuropathy presented with a purely sensory axonal neuropathy, and the disorder was symmetric in 53% of them.
Pathological changes suggestive of vasculitis without frank vasculitic lesions have also been described in some patients with progressive idiopathic axonal neuropathy (Vrancken et al, 2004), and Logigian et al (1993), and Kelkar et al (2002) reported finding focal inflammatory infiltrates without necrosis of blood vessel walls in some patients presenting with sensory neuropathy or multifocal neuropathy that responded to immune therapy.21-23
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) and Other Demyelinating Polyneuropathies

Figure 6. The C3d component of complement in the myelin sheaths of nerve fibers is demonstrated by immunofluorescence. This is accompanied by the presence of IgM at the same site (not shown).
In published nerve biopsy studies, CIDP was diagnosed in 12% of cases reported by Oh (1990), 14% of patients reported by Chia et al (1996), and 5% of cases reported by Schweikert et al (2007). Logigian et al (1994) reported identifying CIDP in 16 of 105 patients (15%) who underwent nerve biopsy.24
The diagnosis of CIDP requires evidence for primary demyelination, which can be provided by nerve conduction studies or pathological examination of nerve biopsy. Electrodiagnostic studies are preferred, as they are minimally invasive, and convenient. However, there is a discrepancy between their sensitivity and specificity, so that the diagnosis of CIDP may sometimes be missed.25,26 In such cases, the demyelination may be detected by nerve biopsy. Biopsy is particularly useful in sensory CIDP (Chin et al, 2004) as electrodiagnostic measures of demyelination are applicable mostly to motor nerves and they cannot be determined in nerves that are unresponsive to stimulation.27 The discrepancy between sensitivity and specificity of electrodiagnostic studies results from a number of factors including: 1) demyelinating changes in sensory nerves are not readily detected, 2) demyelination can occur proximally or distally to the nerve segments examined, 3) demyelinating changes can be masked by axonal degeneration, and 4) early or mild demyelinating changes may not reach diagnostic threshold.28 The latter results from the fact that nerve conduction abnormalities were originally considered to be in the demyelinating range if they exceeded those that can result from axonal degeneration, with axonal neuropathy being the default diagnosis.
Accordingly, Logigian et al (1994) reported that, based on nerve biopsy or nerve conduction studies, the presence of CIDP would have been missed in 5 of 16 patients with CIDP if it weren’t for the nerve biopsy. Of these cases, 3 had sensory CIDP with absent sural responses and sparing of the motor nerves, and 2 had indeterminate studies, with absent responses in the legs. Bosboom et al (2001) reported considerable overlap between nerve biopsy abnormalities in patients classified as having CIDP or chronic axonal neuropathy, but the classification was based on AAN electrodiagnostic demyelinating criteria that have a sensitivity of only 45% (Rajabally et al, 2009), putting their conclusions into question.26,29 Haq et al (2000) reported that sural nerve histologic criteria increased the diagnostic yield when added to the electrodiagnostic criteria for CIDP. Vallat et al (2003) reported that 8 of 44 biopsied patients with pathological diagnosis of CIDP, including 5 that responded to immune therapy, had electrodiagnostic studies that did not meet any of the accepted criteria for demyelination.30 They concluded that a significant number of patients with CIDP are erroneously classified as having axonal neuropathy based on EDX criteria alone. Chin et al (2004), reported 8 patients with sensory neuropathy and axonal or minimally abnormal electrodiagnostic changes, in whom CIDP was diagnosed by sural nerve biopsy.
In addition to distinguishing between demyelination and axonal degeneration, nerve biopsy can help diagnose neuropathy associated with anti-MAG monoclonal IgM antibodies by the presence of IgM and complement on myelin sheaths (Figure 6) and widened myelin lamellae (Figure 4).31 It can also help differentiate between multifocal motor neuropathy and motor neuron disease by the presence of regenerative clusters in motor nerves of patients with multifocal motor neuropathy (Figure 3D).32,33 Studies are ongoing to identify markers that would distinguish between inflammatory and non-inflammatory demyelinating neuropathies.34–36
Other Diagnoses
Amyloid, sarcoidosis, leprosy, or lymphomatosis were found in an occasional patient in several of the series.1,2,8–10 These findings were generally not suspected beforehand. In the series of sarcoid neuropathy by Said et al (2002b), 9 of 11 patients were not known to have sarcoidosis prior to the biopsy. Amyloid neuropathy can result from deposition of immunoglobulin light chain fragments in primary amyloidosis, or of mutated transthyretin, apolipoprotein A-1 or gelsolin in familial forms of amyloidosis. In primary amyloidosis, 90% of patients have a serum or urine monoclonal gammopathy. The type of amyloid deposits can be identified by immunocytochemistry in primary amyloidosis and in familial amyloidotic neuropathy due to a mutation of transthyretin.37–39
Potential Complications of Nerve Biopsy
The rate of complications varied widely between centers. In chronological order, Asbury and Connolly (1973) reported serious side effects in 2 of 103 cases, one with neuroma and one with pain.40 Stevens et al (1975) reported that 10% had significant pain or paresthesias at one year.41 Pollock et al (1983) reported that at 5 years patients only reported non-troublesome and mild dysesthetic symptoms.42 Dyck et al (1984) reported persistent discomfort in 36% and 40% after 3 and 6 months respectively, with 10% having significant pain or paresthesias after one year. Solders et al (1988) reported that immediately after the surgery, approximately half the patients (26/54) experience pain in the operated area.43 In 6/54 (11%) the pain lasted for more than 7 days, and at 6 months 3 of the patients (5.5%) had moderate discomfort and 3 severe discomfort described as a pinprick or “tight” sensation during standing or walking. Some loss of sensation in the foot was reported by 24/54 (44%) of the patients. Neundorfer et al (1990) reported that 53% had sensory deficits, 32% had paresthesias and dysesthesias, and 28% had pain, which in 25% was present at 21 months. Oh (1990) reported that 0.5% had neuralgia lasting one year. Gabriel et al (2000) reported that at 6 months, 20% reported pain at the biopsy site. Dahlin et al (1997) reported that 40% of patients with diabetes but none of the patients without diabetes reported pain in the biopsy site at 20 to 44 months after the biopsy.44 Theriault et al (1998) reported that at 12 months, 19% had allodynia that was rated as 3 on a scale of 1 to 10.45 Said (2002a) recommended immobilizing the leg in a plastic cast for 10 days following surgery to prevent excessive tension.
- Oh S. Diagnostic usefulness and limitations of the sural nerve biopsy. Yonsei Med J. 1990;31(1):1-26. [PubMed]
- Midroni G, Bilbao J. Nerve Biopsy. In: Peripheral Nerve Diseases, Handbook of Clinical Neurophysiology. Vol 7. Elsevier BV; 2006:63-94.
- Schröder J. Recommendations for the examination of peripheral nerve biopsies. Virchows Arch. 1998;432(3):199-205. [PubMed]
- Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59(10):1532-1535. [PubMed]
- Ruth A, Schulmeyer F, Roesch M, Woertgen C, Brawanski A. Diagnostic and therapeutic value due to suspected diagnosis, long-term complications, and indication for sural nerve biopsy. Clin Neurol Neurosurg. 2005;107(3):214-217. [PubMed]
- Bevilacqua N, Rogers L, Malik R, Armstrong D. Technique of the sural nerve biopsy. J Foot Ankle Surg. 2007;46(2):139-142. [PubMed]
- Neundörfer B, Grahmann F, Engelhardt A, Harte U. Postoperative effects and value of sural nerve biopsies: a retrospective study. Eur Neurol. 1990;30(6):350-352. [PubMed]
- Chia L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain. 1996;119 ( Pt 4):1091-1098. [PubMed]
- Gabriel C, Howard R, Kinsella N, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry. 2000;69(4):442-446. [PubMed]
- Schweikert K, Fuhr P, Probst A, Tolnay M, Renaud S, Steck A. Contribution of nerve biopsy to unclassified neuropathy. Eur Neurol. 2007;57(2):86-90. [PubMed]
- Seo J, Ryan H, Claussen G, Thomas T, Oh S. Sensory neuropathy in vasculitis: a clinical, pathologic, and electrophysiologic study. Neurology. 2004;63(5):874-878. [PubMed]
- Vital C, Vital A, Canron M, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst. 2006;11(1):20-29. [PubMed]
- Vrancken A, Gathier C, Cats E, Notermans N, Collins M. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18(1):49-58. [PubMed]
- Bennett D, Groves M, Blake J, et al. The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry. 2008;79(12):1376-1381. [PubMed]
- Collins M. Localized vasculitis and the peripheral nervous system. J Rheumatol. 2005;32(5):769-772. [PubMed]
- Collins M, Mendell J, Periquet M, et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55(5):636-643. [PubMed]
- Sugiura M, Koike H, Iijima M, et al. Clinicopathologic features of nonsystemic vasculitic neuropathy and microscopic polyangiitis-associated neuropathy: a comparative study. J Neurol Sci. 2006;241(1-2):31-37. [PubMed]
- Burns T, Schaublin G, Dyck P. Vasculitic neuropathies. Neurol Clin. 2007;25(1):89-113. [PubMed]
- Gorson K. Vasculitic neuropathies: an update. Neurologist. 2007;13(1):12-19. [PubMed]
- Zivković S, Ascherman D, Lacomis D. Vasculitic neuropathy–electrodiagnostic findings and association with malignancies. Acta Neurol Scand. 2007;115(6):432-436. [PubMed]
- Vrancken A, Notermans N, Jansen G, Wokke J, Said G. Progressive idiopathic axonal neuropathy–a comparative clinical and histopathological study with vasculitic neuropathy. J Neurol. 2004;251(3):269-278. [PubMed]
- Logigian E, Shefner J, Frosch M, et al. Nonvasculitic, steroid-responsive mononeuritis multiplex. Neurology. 1993;43(5):879-883. [PubMed]
- Kelkar P, McDermott W, Parry G. Sensory-predominant, painful, idiopathic neuropathy: inflammatory changes in sural nerves. Muscle Nerve. 2002;26(3):413-416. [PubMed]
- Logigian E, Kelly J, Adelman L. Nerve conduction and biopsy correlation in over 100 consecutive patients with suspected polyneuropathy. Muscle Nerve. 1994;17(9):1010-1020. [PubMed]
- De S, Chin R, Sander H, Latov N, Brannagan T. Demyelinating findings in typical and atypical chronic inflammatory demyelinating polyneuropathy: sensitivity and specificity. J Clin Neuromuscul Dis. 2009;10(4):163-169. [PubMed]
- Rajabally Y, Nicolas G, Piéret F, Bouche P, Van den. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: a multicentre European study. J Neurol Neurosurg Psychiatry. 2009;80(12):1364-1368. [PubMed]
- Chin R, Latov N, Sander H, et al. Sensory CIDP presenting as cryptogenic sensory polyneuropathy. J Peripher Nerv Syst. 2004;9(3):132-137. [PubMed]
- Krarup C, Trojaborg W. Sensory pathophysiology in chronic acquired demyelinating neuropathy. Brain. 1996;119 ( Pt 1):257-270. [PubMed]
- Bosboom W, van den, Franssen H, et al. Diagnostic value of sural nerve demyelination in chronic inflammatory demyelinating polyneuropathy. Brain. 2001;124(Pt 12):2427-2438. [PubMed]
- Vallat J, Tabaraud F, Magy L, et al. Diagnostic value of nerve biopsy for atypical chronic inflammatory demyelinating polyneuropathy: evaluation of eight cases. Muscle Nerve. 2003;27(4):478-485. [PubMed]
- Takatsu M, Hays AP, Latov N, et al. Immunofluorescence study of patients with neuropathy and IgM M proteins. A. 1985;18(2):173-181. doi:10.1002/ana.410180203
- Corbo M, Abouzahr M, Latov N, et al. Motor nerve biopsy studies in motor neuropathy and motor neuron disease. Muscle Nerve. 1997;20(1):15-21. [PubMed]
- Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1):197-201. [PubMed]
- Renaud S, Hays A, Brannagan T, et al. Gene expression profiling in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2005;159(1-2):203-214. [PubMed]
- Sommer C, Brandner S, Dyck P, et al. 147th ENMC international workshop: guideline on processing and evaluation of sural nerve biopsies, 15-17 December 2006, Naarden, The Netherlands. Neuromuscul Disord. 2008;18(1):90-96. [PubMed]
- Kinter J, Broglio L, Steck A, et al. Gene expression profiling in nerve biopsy of vasculitic neuropathy. J Neuroimmunol. 2010;225(1-2):184-189. [PubMed]
- Kissel J, Mendell J. Neuropathies associated with monoclonal gammopathies. Neuromuscul Disord. 1996;6(1):3-18. [PubMed]
- Matsuda M, Gono T, Morita H, Katoh N, Kodaira M, Ikeda S. Peripheral nerve involvement in primary systemic AL amyloidosis: a clinical and electrophysiological study. Eur J Neurol. 2011;18(4):604-610. [PubMed]
- Cappellari M, Cavallaro T, Ferrarini M, et al. Variable presentations of TTR-related familial amyloid polyneuropathy in seventeen patients. J Peripher Nerv Syst. 2011;16(2):119-129. [PubMed]
- Asbury A, Connolly E. Sural nerve biopsy. Technical note. J Neurosurg. 1973;38(3):391-392. [PubMed]
- Stevens J, Lofgren E, Dyck P. Biopsy of peripheral nerves. In: Peripheral Neuropathy. Vol 1. Philadelphia: Saunders; 1975:410-423.
- Pollock M, Nukada H, Taylor P, Donaldson I, Carroll G. Comparison between fascicular and whole sural nerve biopsy. Ann Neurol. 1983;13(1):65-68. [PubMed]
- Solders G. Discomfort after fascicular sural nerve biopsy. Acta Neurol Scand. 1988;77(6):503-504. [PubMed]
- Dahlin L, Eriksson K, Sundkvist G. Persistent postoperative complaints after whole sural nerve biopsies in diabetic and non-diabetic subjects. Diabet Med. 1997;14(5):353-356. [PubMed]
- Theriault M, Dort J, Sutherland G, Zochodne D. A prospective quantitative study of sensory deficits after whole sural nerve biopsies in diabetic and nondiabetic patients. Surgical approach and the role of collateral sprouting. Neurology. 1998;50(2):480-484. [PubMed]
Related Articles
Nerve Pathology Overview
Becky Tsai2022-09-04T01:43:54-05:00
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy... [Read More]
Muscle Pathology Overview
Becky Tsai2022-09-04T14:24:50-05:00
Muscle biopsy is used to confirm that there is a morphological or biochemical abnormality that correlates with or could account for clinical symptoms. [Read More]
Therapath Neuropathology Announces National Ophthalmic Pathology Program
Becky Tsai2022-09-05T17:49:10-05:00
Therapath LLC, a leader in neuropathology laboratory services, launches its national ophthalmic pathology program. [Read More]
Nerve Pathology Overview
Indications for Nerve Biopsy
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy, when other tests fail to diagnose these conditions. Given that it is an invasive test, however, a nerve biopsy is usually only recommended when the neuropathy is progressive, threatens to become debilitating, and other tests fail to identify a cause.1–3 Vasculitis, amyloidosis, sarcoid and other multifocal disorders may also affect skeletal muscle so that biopsy of muscle in addition to nerve may increase the diagnostic yield.
Location of the Nerve Biopsy
In cases where the neuropathy affects the lower limbs, the superficial peroneal nerve with the peroneus brevis muscle, or the sural nerve with the vastus lateralis or gastrocnemius muscle are usually examined. When only the upper limbs are affected, the superficial radial nerve or a branch of the ulnar nerve in the dorsum of the hand can be biopsied.4–6 Targeted biopsy of a sensory rootlet in demyelinating sensory radiculopathy, or of the obturator nerve branch to the gracilis muscle in multifocal motor neuropathy have also been described.
Pathological Examination
The biopsy nerve samples are prepared in three different ways to fully assess the tissue for diagnostic purposes. One nerve piece (0.5 cm in length) is fixed in 10% formalin and embedded in paraffin; a second piece of similar length is transported in Michel’s medium and frozen in the laboratory using liquid nitrogen; and a third piece (2.0 to 2.5 in length) is placed in glutaraldehyde fixative for preparation of semithin plastic sections and teased myelinated nerve fibers.

Figure 1. Necrotizing arteritis, paraffin section, H&E.
In general, light microscopic examination of formalin-fixed, paraffin-embedded tissue is the most important method for establishing a definitive diagnosis such as vasculitis (Figure 1), amyloidosis (Figure 2A), sarcoidosis, leprosy and lymphoma. If the initial microscopic examination is not informative and these disorders are suspected based on clinical or pathological findings, more extensive sampling of the tissue is indicated to search for a focal definitive lesion. In suspected vasculitis, special stains for the internal elastica of arteries and peri-arterial hemosiderin can provide indirect evidence for the diagnosis in the absence of a definitive lesion, such as inflammatory infiltration of the blood vessel wall or fibrinoid necrosis. All nerves and muscles should be screened by the thioflavin S stain to identify amyloid deposits (Figure 2B). This stain is more sensitive than Congo red for detecting amyloid, but is less specific and requires the Congo red stain to confirm the diagnosis (Figure 2C, 2D). Amyloid can be further subclassified by immunohistochemistry to identify transthyretin or immunoglobulin light chains that cause the two chief types of amyloidotic neuropathy. The immunoreactive transthyretin can be detected in paraffin sections, but staining of light chains requires sections prepared from unfixed frozen tissue. In general, lymphocytic markers are not necessary for diagnosis except for a suspected lymphoma or other lymphoproliferative disorder of nerve.

Figure 2. A: Amyloid deposits (arrows) in endoneurial compartment of nerve, cryosection, H&E. B: Thioflavin S bound to amyloid deposits (arrows), paraffin section (cross-section), fluorescence. C: Amyloid deposits (arrows), paraffin section, Congo Red. D: Outlined area in C enlarged and viewed with crossed polarizing lenses showing greenish birefringence (arrows) in amyloid deposits
Transverse semithin plastic sections of nerve (Figure 3A-D) are more useful for analysis of myelinated fibers than the thicker paraffin sections because of better definition for assessing small myelinated fibers, onion bulbs and other structures. The myelin sheaths in the sections are stained by osmium tetroxide in a pre-embedding step and stained by toluidine blue in a post-embedding step. The findings help to provide a distinction between a purely axonal disorder and primary demyelination, particularly in the acute phase. The number of myelinated fibers per unit area of endoneurium can be quantified, and the result can indicate a loss of myelinated fibers in comparison to reference values if necessary. Reduction of the number of myelinated fibers is usually caused by axonal loss with secondary degeneration of myelin sheaths. The secondary myelin breakdown may be prominent in the acute stage. In the chronic stage, the number of regenerative clusters of three or more small myelinated fibers may be excessive (Figure 3D) and provide further evidence for a chronic axonal process.

Figure 3. Transverse plastic semithin sections of nerve. A: Whole normal sural nerve, toluidine blue. B: Higher magnification of A. The number of small diameter myelinated fibers is roughly twice that of large fibers. C: Neuropathy with an IgM paraprotein reactive to myelin-associated glycoprotein (sural nerve, toluidine blue). There is a loss of myelinated fibers and scattered isolated thinly myelinated fibers, probably reflecting segmental remyelination. A few onion bulbs are observed (arrows). D: Multifocal motor neuropathy. A transverse section of the motor nerve of the gracilis muscle displays large regenerative clusters of small myelinated fibers (arrows) (toluidine blue-basic fuchsin).
Acute primary demyelination of nerve is represented by large “naked” axons that lack myelin sheaths and are often encircled by myelin debris-containing macrophages. Additional evidence for demyelination can be provided by electron microscopy studies or distinctive ultrastructural changes such as widened myelin lamellae in neuropathy associated with monoclonal IgM anti-MAG (myelin-associated glycoprotein) antibodies (Figure 4). Months after the clinical onset, onion bulbs may appear in transverse sections (Figure 3C). The abnormality consists of concentrically oriented Schwann cell processes that surround a thinly-myelinated fiber as a result of segmental remyelination. Segmental remyelination appears more often without onion bulb formation, but it cannot be distinguished reliably from fibers that have undergone axonal regeneration and remyelination in semithin sections. This distinction requires teased myelinated fiber analysis (Figure 5), which is more sensitive than semithin sections for identifying demyelinating and axonal features. Segmental demyelination, segmental remyelination and tomacula (focally thickened myelin sheaths) are characteristic features of a demyelinating disorder, whereas myelin breakdown into ovoids along the full length of a teased fiber indicates axonal degeneration. The analysis requires quantifying the abnormalities in a minimum of 50 teased myelinated fibers with comparison to the data of age-matched normal subjects. However, the teased fiber results in chronic neuropathies can be difficult to interpret because demyelination can result from either primary attack of the myelin sheath or a secondary effect of axonal dysfunction.

Figure 5. A: A single teased myelinated fiber shows segmental remyelination in the form of three short segments (arrows) of myelin flanked by normal internodes (osmium tetroxide). B: Higher magnification of outlined area in A
Diagnostic Yield
Several studies have been published that reviewed the authors’ experience with nerve biopsies. Argov et al (1989) reported that the nerve biopsy contributed to the final diagnosis in 18% of 120 biopsies. Neundorfer et al (1990) reported that the diagnosis was made on basis of the biopsy alone in 27% of 56 patients and that it contributed valuable diagnostic information in 37%.7 Oh (1990) reported that specific diagnoses were made in 24% of 385 cases, and that the biopsy provided helpful information in 45%. Chia et al (1996) reported that the biopsy was necessary for diagnosis in 33% of 100 elderly patients over the age of 65.8 Gabriel et al (2000) reported that the biopsy changed the diagnosis in 14% of 50 cases, and was helpful in an additional 70% as it confirmed the suspected diagnosis.9 Midroni and Bilboa (2006) reported that the biopsy was essential for patient management in 21% of 234 cases. Schweikert et al (2007) reported that the biopsy was diagnostic in 16% of 38 patients, and supportive of the suspected diagnosis in 21%.10 The differences between studies probably resulted from differences in patient selection between the reporting centers.
Vasculitis
Vasculitis was by far the most common diagnosis made on the basis of nerve biopsy (Figure 1). Vasculitis was identified in 12% of biopsies reported by Oh (1990), 8% of patients reported by Midroni and Bilbao (2006), 16% of cases reported by Schweikert et al (2007), and 33% of elderly patients reported by Chia et al (1996).
Most authors found that doing a muscle biopsy added to the diagnostic yield. Chia et al (1996) reported that of the patients with vasculitis, 43% had vasculitic changes in both nerve and muscle, 65% in muscle, and 78% in nerves. Schroder et al (1998) reported that of 129 cases of vasculitis, both muscle and nerve were positive in 27%, nerve only in 30%, and muscle only in 27%. Seo et al (2004) reported that the muscle biopsy was necessary for diagnosis in 12% of their patients.11 Vital et al (2006) reported that muscle biopsy improved the yield of definite vasculitis by 27%.12 Vrancken et al (2010) reported that in patients diagnosed with vasculitis, the additional yield of muscle biopsy was 15%.13 Bennett et al (2008), reported that combined sural nerve and vastus lateralis muscle biopsy did not significantly increase the diagnostic yield compared to nerve biopsy alone.14 However, they recommended that if the superficial peroneal nerve is chosen, then it would be appropriate to also biopsy the peroneus brevis muscle.
Clinically, vasculitis in neuropathy can be systemic and involve other organs, or nonsystemic in which case it is clinically localized to peripheral nerves (Figure 1). In systemic vasculitis, the diagnosis can be based on biopsy findings of any affected organ, but in nonsystemic vasculitic neuropathy, the diagnosis is made on the basis of nerve and muscle biopsy.15–19 Patients presented with mononeuritis multiplex or diffuse polyneuropathy in an asymmetric or distal and symmetric distribution, probably resulting from a confluence of lesions. In a large series of nonsystemic vasculitic neuropathy, asymmetric findings were reported in 59% to 98% of cases, with pain being a prominent feature in 75% to 98%.16
In the series by Chia et al (1996), 23% of biopsies had a necrotizing arteritis, and 15% had inflammatory infiltrates in the vicinity of nerve and muscle blood vessels associated with axonal degeneration and multifocal neuropathy. In 20 of 33 patients, the neuropathy occurred in the context of a multisystem disorder and in 13 the biopsy was the only indication of vasculitis.
In electrophysiologic studies, Zivkovic et al (2007) reported that the most common presentation of patients with vasculitic neuropathy was generalized neuropathy with electrodiagnosis showing a pattern of mononeuritis multiplex in 27.5%, and axonal sensorimotor neuropathy with side to side amplitude asymmetry in 50%, with the remaining showing a symmetric axonal neuropathy.20 Seo et al (2004) reported that 16 of their 106 patients (16%) with vasculitic neuropathy presented with a purely sensory axonal neuropathy, and the disorder was symmetric in 53% of them.
Pathological changes suggestive of vasculitis without frank vasculitic lesions have also been described in some patients with progressive idiopathic axonal neuropathy (Vrancken et al, 2004), and Logigian et al (1993), and Kelkar et al (2002) reported finding focal inflammatory infiltrates without necrosis of blood vessel walls in some patients presenting with sensory neuropathy or multifocal neuropathy that responded to immune therapy.21-23
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) and Other Demyelinating Polyneuropathies

Figure 6. The C3d component of complement in the myelin sheaths of nerve fibers is demonstrated by immunofluorescence. This is accompanied by the presence of IgM at the same site (not shown).
In published nerve biopsy studies, CIDP was diagnosed in 12% of cases reported by Oh (1990), 14% of patients reported by Chia et al (1996), and 5% of cases reported by Schweikert et al (2007). Logigian et al (1994) reported identifying CIDP in 16 of 105 patients (15%) who underwent nerve biopsy.24
The diagnosis of CIDP requires evidence for primary demyelination, which can be provided by nerve conduction studies or pathological examination of nerve biopsy. Electrodiagnostic studies are preferred, as they are minimally invasive, and convenient. However, there is a discrepancy between their sensitivity and specificity, so that the diagnosis of CIDP may sometimes be missed.25,26 In such cases, the demyelination may be detected by nerve biopsy. Biopsy is particularly useful in sensory CIDP (Chin et al, 2004) as electrodiagnostic measures of demyelination are applicable mostly to motor nerves and they cannot be determined in nerves that are unresponsive to stimulation.27 The discrepancy between sensitivity and specificity of electrodiagnostic studies results from a number of factors including: 1) demyelinating changes in sensory nerves are not readily detected, 2) demyelination can occur proximally or distally to the nerve segments examined, 3) demyelinating changes can be masked by axonal degeneration, and 4) early or mild demyelinating changes may not reach diagnostic threshold.28 The latter results from the fact that nerve conduction abnormalities were originally considered to be in the demyelinating range if they exceeded those that can result from axonal degeneration, with axonal neuropathy being the default diagnosis.
Accordingly, Logigian et al (1994) reported that, based on nerve biopsy or nerve conduction studies, the presence of CIDP would have been missed in 5 of 16 patients with CIDP if it weren’t for the nerve biopsy. Of these cases, 3 had sensory CIDP with absent sural responses and sparing of the motor nerves, and 2 had indeterminate studies, with absent responses in the legs. Bosboom et al (2001) reported considerable overlap between nerve biopsy abnormalities in patients classified as having CIDP or chronic axonal neuropathy, but the classification was based on AAN electrodiagnostic demyelinating criteria that have a sensitivity of only 45% (Rajabally et al, 2009), putting their conclusions into question.26,29 Haq et al (2000) reported that sural nerve histologic criteria increased the diagnostic yield when added to the electrodiagnostic criteria for CIDP. Vallat et al (2003) reported that 8 of 44 biopsied patients with pathological diagnosis of CIDP, including 5 that responded to immune therapy, had electrodiagnostic studies that did not meet any of the accepted criteria for demyelination.30 They concluded that a significant number of patients with CIDP are erroneously classified as having axonal neuropathy based on EDX criteria alone. Chin et al (2004), reported 8 patients with sensory neuropathy and axonal or minimally abnormal electrodiagnostic changes, in whom CIDP was diagnosed by sural nerve biopsy.
In addition to distinguishing between demyelination and axonal degeneration, nerve biopsy can help diagnose neuropathy associated with anti-MAG monoclonal IgM antibodies by the presence of IgM and complement on myelin sheaths (Figure 6) and widened myelin lamellae (Figure 4).31 It can also help differentiate between multifocal motor neuropathy and motor neuron disease by the presence of regenerative clusters in motor nerves of patients with multifocal motor neuropathy (Figure 3D).32,33 Studies are ongoing to identify markers that would distinguish between inflammatory and non-inflammatory demyelinating neuropathies.34–36
Other Diagnoses
Amyloid, sarcoidosis, leprosy, or lymphomatosis were found in an occasional patient in several of the series.1,2,8–10 These findings were generally not suspected beforehand. In the series of sarcoid neuropathy by Said et al (2002b), 9 of 11 patients were not known to have sarcoidosis prior to the biopsy. Amyloid neuropathy can result from deposition of immunoglobulin light chain fragments in primary amyloidosis, or of mutated transthyretin, apolipoprotein A-1 or gelsolin in familial forms of amyloidosis. In primary amyloidosis, 90% of patients have a serum or urine monoclonal gammopathy. The type of amyloid deposits can be identified by immunocytochemistry in primary amyloidosis and in familial amyloidotic neuropathy due to a mutation of transthyretin.37–39
Potential Complications of Nerve Biopsy
The rate of complications varied widely between centers. In chronological order, Asbury and Connolly (1973) reported serious side effects in 2 of 103 cases, one with neuroma and one with pain.40 Stevens et al (1975) reported that 10% had significant pain or paresthesias at one year.41 Pollock et al (1983) reported that at 5 years patients only reported non-troublesome and mild dysesthetic symptoms.42 Dyck et al (1984) reported persistent discomfort in 36% and 40% after 3 and 6 months respectively, with 10% having significant pain or paresthesias after one year. Solders et al (1988) reported that immediately after the surgery, approximately half the patients (26/54) experience pain in the operated area.43 In 6/54 (11%) the pain lasted for more than 7 days, and at 6 months 3 of the patients (5.5%) had moderate discomfort and 3 severe discomfort described as a pinprick or “tight” sensation during standing or walking. Some loss of sensation in the foot was reported by 24/54 (44%) of the patients. Neundorfer et al (1990) reported that 53% had sensory deficits, 32% had paresthesias and dysesthesias, and 28% had pain, which in 25% was present at 21 months. Oh (1990) reported that 0.5% had neuralgia lasting one year. Gabriel et al (2000) reported that at 6 months, 20% reported pain at the biopsy site. Dahlin et al (1997) reported that 40% of patients with diabetes but none of the patients without diabetes reported pain in the biopsy site at 20 to 44 months after the biopsy.44 Theriault et al (1998) reported that at 12 months, 19% had allodynia that was rated as 3 on a scale of 1 to 10.45 Said (2002a) recommended immobilizing the leg in a plastic cast for 10 days following surgery to prevent excessive tension.
- Oh S. Diagnostic usefulness and limitations of the sural nerve biopsy. Yonsei Med J. 1990;31(1):1-26.[PubMed]
- Midroni G, Bilbao J. Nerve Biopsy. In: Peripheral Nerve Diseases, Handbook of Clinical Neurophysiology. Vol 7. Elsevier BV; 2006:63-94.
- Schröder J. Recommendations for the examination of peripheral nerve biopsies. Virchows Arch. 1998;432(3):199-205.[PubMed]
- Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59(10):1532-1535.[PubMed]
- Ruth A, Schulmeyer F, Roesch M, Woertgen C, Brawanski A. Diagnostic and therapeutic value due to suspected diagnosis, long-term complications, and indication for sural nerve biopsy. Clin Neurol Neurosurg. 2005;107(3):214-217.[PubMed]
- Bevilacqua N, Rogers L, Malik R, Armstrong D. Technique of the sural nerve biopsy. J Foot Ankle Surg. 2007;46(2):139-142.[PubMed]
- Neundörfer B, Grahmann F, Engelhardt A, Harte U. Postoperative effects and value of sural nerve biopsies: a retrospective study. Eur Neurol. 1990;30(6):350-352.[PubMed]
- Chia L, Fernandez A, Lacroix C, Adams D, Planté V, Said G. Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain. 1996;119 ( Pt 4):1091-1098.[PubMed]
- Gabriel C, Howard R, Kinsella N, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry. 2000;69(4):442-446.[PubMed]
- Schweikert K, Fuhr P, Probst A, Tolnay M, Renaud S, Steck A. Contribution of nerve biopsy to unclassified neuropathy. Eur Neurol. 2007;57(2):86-90.[PubMed]
- Seo J, Ryan H, Claussen G, Thomas T, Oh S. Sensory neuropathy in vasculitis: a clinical, pathologic, and electrophysiologic study. Neurology. 2004;63(5):874-878.[PubMed]
- Vital C, Vital A, Canron M, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst. 2006;11(1):20-29.[PubMed]
- Vrancken A, Gathier C, Cats E, Notermans N, Collins M. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur J Neurol. 2011;18(1):49-58.[PubMed]
- Bennett D, Groves M, Blake J, et al. The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry. 2008;79(12):1376-1381.[PubMed]
- Collins M. Localized vasculitis and the peripheral nervous system. J Rheumatol. 2005;32(5):769-772.[PubMed]
- Collins M, Mendell J, Periquet M, et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55(5):636-643.[PubMed]
- Sugiura M, Koike H, Iijima M, et al. Clinicopathologic features of nonsystemic vasculitic neuropathy and microscopic polyangiitis-associated neuropathy: a comparative study. J Neurol Sci. 2006;241(1-2):31-37.[PubMed]
- Burns T, Schaublin G, Dyck P. Vasculitic neuropathies. Neurol Clin. 2007;25(1):89-113.[PubMed]
- Gorson K. Vasculitic neuropathies: an update. Neurologist. 2007;13(1):12-19.[PubMed]
- Zivković S, Ascherman D, Lacomis D. Vasculitic neuropathy–electrodiagnostic findings and association with malignancies. Acta Neurol Scand. 2007;115(6):432-436.[PubMed]
- Vrancken A, Notermans N, Jansen G, Wokke J, Said G. Progressive idiopathic axonal neuropathy–a comparative clinical and histopathological study with vasculitic neuropathy. J Neurol. 2004;251(3):269-278.[PubMed]
- Logigian E, Shefner J, Frosch M, et al. Nonvasculitic, steroid-responsive mononeuritis multiplex. Neurology. 1993;43(5):879-883.[PubMed]
- Kelkar P, McDermott W, Parry G. Sensory-predominant, painful, idiopathic neuropathy: inflammatory changes in sural nerves. Muscle Nerve. 2002;26(3):413-416.[PubMed]
- Logigian E, Kelly J, Adelman L. Nerve conduction and biopsy correlation in over 100 consecutive patients with suspected polyneuropathy. Muscle Nerve. 1994;17(9):1010-1020.[PubMed]
- De S, Chin R, Sander H, Latov N, Brannagan T. Demyelinating findings in typical and atypical chronic inflammatory demyelinating polyneuropathy: sensitivity and specificity. J Clin Neuromuscul Dis. 2009;10(4):163-169.[PubMed]
- Rajabally Y, Nicolas G, Piéret F, Bouche P, Van den. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: a multicentre European study. J Neurol Neurosurg Psychiatry. 2009;80(12):1364-1368.[PubMed]
- Chin R, Latov N, Sander H, et al. Sensory CIDP presenting as cryptogenic sensory polyneuropathy. J Peripher Nerv Syst. 2004;9(3):132-137.[PubMed]
- Krarup C, Trojaborg W. Sensory pathophysiology in chronic acquired demyelinating neuropathy. Brain. 1996;119 ( Pt 1):257-270.[PubMed]
- Bosboom W, van den, Franssen H, et al. Diagnostic value of sural nerve demyelination in chronic inflammatory demyelinating polyneuropathy. Brain. 2001;124(Pt 12):2427-2438.[PubMed]
- Vallat J, Tabaraud F, Magy L, et al. Diagnostic value of nerve biopsy for atypical chronic inflammatory demyelinating polyneuropathy: evaluation of eight cases. Muscle Nerve. 2003;27(4):478-485.[PubMed]
- Takatsu M, Hays AP, Latov N, et al. Immunofluorescence study of patients with neuropathy and IgM M proteins. A. 1985;18(2):173-181.
doi:10.1002/ana.410180203
- Corbo M, Abouzahr M, Latov N, et al. Motor nerve biopsy studies in motor neuropathy and motor neuron disease. Muscle Nerve. 1997;20(1):15-21.[PubMed]
- Riva N, Iannaccone S, Corbo M, et al. Motor nerve biopsy: clinical usefulness and histopathological criteria. Ann Neurol. 2011;69(1):197-201.[PubMed]
- Renaud S, Hays A, Brannagan T, et al. Gene expression profiling in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2005;159(1-2):203-214.[PubMed]
- Sommer C, Brandner S, Dyck P, et al. 147th ENMC international workshop: guideline on processing and evaluation of sural nerve biopsies, 15-17 December 2006, Naarden, The Netherlands. Neuromuscul Disord. 2008;18(1):90-96.[PubMed]
- Kinter J, Broglio L, Steck A, et al. Gene expression profiling in nerve biopsy of vasculitic neuropathy. J Neuroimmunol. 2010;225(1-2):184-189.[PubMed]
- Kissel J, Mendell J. Neuropathies associated with monoclonal gammopathies. Neuromuscul Disord. 1996;6(1):3-18.[PubMed]
- Matsuda M, Gono T, Morita H, Katoh N, Kodaira M, Ikeda S. Peripheral nerve involvement in primary systemic AL amyloidosis: a clinical and electrophysiological study. Eur J Neurol. 2011;18(4):604-610.[PubMed]
- Cappellari M, Cavallaro T, Ferrarini M, et al. Variable presentations of TTR-related familial amyloid polyneuropathy in seventeen patients. J Peripher Nerv Syst. 2011;16(2):119-129.[PubMed]
- Asbury A, Connolly E. Sural nerve biopsy. Technical note. J Neurosurg. 1973;38(3):391-392.[PubMed]
- Stevens J, Lofgren E, Dyck P. Biopsy of peripheral nerves. In: Peripheral Neuropathy. Vol 1. Philadelphia: Saunders; 1975:410-423.
- Pollock M, Nukada H, Taylor P, Donaldson I, Carroll G. Comparison between fascicular and whole sural nerve biopsy. Ann Neurol. 1983;13(1):65-68.[PubMed]
- Solders G. Discomfort after fascicular sural nerve biopsy. Acta Neurol Scand. 1988;77(6):503-504.[PubMed]
- Dahlin L, Eriksson K, Sundkvist G. Persistent postoperative complaints after whole sural nerve biopsies in diabetic and non-diabetic subjects. Diabet Med. 1997;14(5):353-356.[PubMed]
- Theriault M, Dort J, Sutherland G, Zochodne D. A prospective quantitative study of sensory deficits after whole sural nerve biopsies in diabetic and nondiabetic patients. Surgical approach and the role of collateral sprouting. Neurology. 1998;50(2):480-484.[PubMed]
Related Articles
Nerve Pathology Overview
Becky Tsai2022-09-04T01:43:54-05:00
Nerve biopsy can identify potentially treatable causes of neuropathy, such as vasculitis, atypical CIDP, sarcoidosis, amyloidosis, lymphomatosis, or leprosy... [Read More]
Muscle Pathology Overview
Becky Tsai2022-09-04T14:24:50-05:00
Muscle biopsy is used to confirm that there is a morphological or biochemical abnormality that correlates with or could account for clinical symptoms. [Read More]
Therapath Neuropathology Announces National Ophthalmic Pathology Program
Becky Tsai2022-09-05T17:49:10-05:00
Therapath LLC, a leader in neuropathology laboratory services, launches its national ophthalmic pathology program. [Read More]

